Can a Higher Density Material Float on a Lower Density Material
Objectives
-
Demonstrate how the distribution of molecules in a substance determines its density.
-
Investigate the relative densities of liquids and the relative densities of solids.
-
Predict, test and explicate relative density past investigating the interactions of liquids and solids.
-
Demonstrate understanding of the human relationship between density and buoyancy by building a boat.
Materials
-
see individual activities for materials.
Background
Density, Mass & Book
Simply put, density is how tightly "stuff" is packed into a defined space.
For example, a suitcase jam-packed with wearing apparel and souvenirs has a high density, while the same suitcase containing 2 pairs of underwear has depression density. Size-wise, both suitcases look the same, but their density depends on the relationship between their mass and book.
Mass is the amount of matter in an object.
Volume is the corporeality of space that an object takes up in three dimensions.
Density is calculated using the following equation: Density = mass/volume or D = m/5.
Let's compare 3 familiar substances to explore the concept of density. If we have the same book (ane cubic centimetre) of foam, wood and concrete, we can see that each has a dissimilar mass.
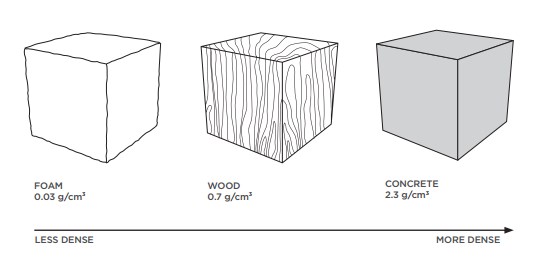
Less Dense, More Dumbo
If something is heavy for its size, it has a high density. If an object is light for its size it has a low density.
A pebble is heavy for its size, compared to a slice of popcorn which is lite for it'south size. Imagine a large basin of popcorn, compared to a big bowl of pebbles, which would feel heavier?
Information technology is easy to estimate relative densities if you keep either the volume or the mass of two objects the same.
If you filled ane bag with a kg of feathers and another with a kg of lead you would see that the feathers take up much more room, fifty-fifty though both numberless take the same mass. This considering feathers are less dense, they accept less mass per volume. If you lot made a copper cube and an aluminum cube of the aforementioned volume and placed one in each paw, y'all would be able to feel that the copper cube would be heavier. Copper has more mass per volume than aluminum.
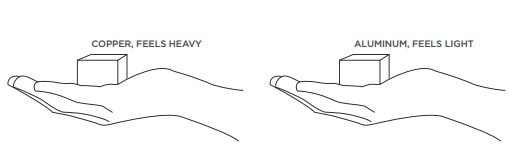
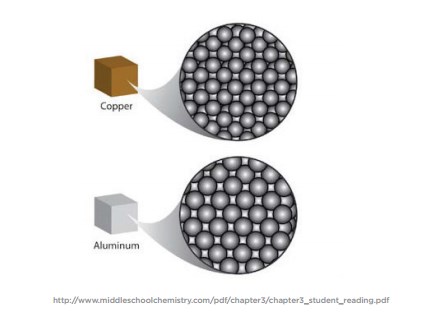
How tin can 1 substance have more mass per volume than another? There are a few possibilities:
- Atoms of one substance might be a similar size yet have more mass than the atoms of another substance.
- Atoms of one substance might be a similar mass but be smaller, so more of them fit within the same volume.
- Atoms of i substance might be bundled in a way that allows more than of them to fit in the same volume.
Whatever ane or a combination of these explanations could exist the reason why ane substance has a higher density than another. In the case of copper and aluminum, their atoms are arranged similarly, but copper atoms are smaller and take more than mass than aluminum atoms, giving information technology a higher density.
Density, Sinking and Floating
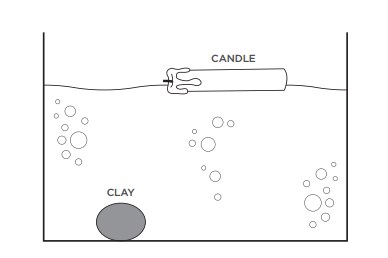
Why do some things float, while others sink? You might expect heavier objects to sink and lighter ones to float, only sometimes the opposite is true. The relative densities of an object and the liquid it is placed in determine whether that object will sink or float. An object that has a higher density than the liquid information technology'southward in will sink. An object that has a lower density than the liquid it's in volition float.
You tin really meet relative densities at piece of work when y'all look at a heavy object floating and a lighter ane sinking. For example, imagine putting a minor piece of clay and a large, heavy wax candle in a tub of water. Fifty-fifty though it'south lighter, the piece of clay has a higher density than water and therefore sinks. Even though it'southward heavier, wax has a lower density than water, so the big candle floats.
Sinking and floating applies to liquids too. For example, if y'all add together vegetable oil to h2o, the oil floats on height of the h2o considering the oil has a lower density than the water.
Buoyancy and Archimedes' Principle
The aboriginal Greek philosopher Archimedes establish that when an object is submerged in water, information technology pushes aside (or displaces) an amount of water with the aforementioned mass as the object.
The water pushes upward against the object with a strength (buoyancy) equal to the weight of water that is displaced.
Permit's explore Archimedes' principle by dropping a bowling ball into a tub of water. When the brawl is submerged in the water, it displaces its book in water. According to Archimedes' principle, the water can "button back" with a force equal to the weight of the water that has been displaced.
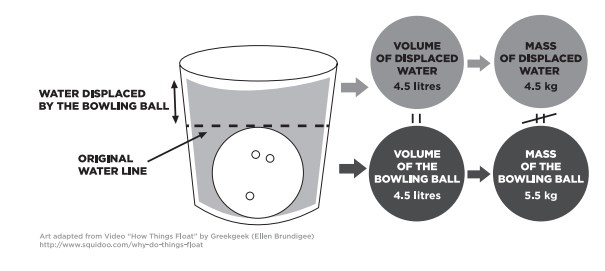
A litre of h2o has a density of 1 kilogram per litre (1 kg/L), so a bowling brawl's worth of water (four.5 L) can push back on the bowling brawl with a force equal to 45 newtons (Due north). That's the weight of a 4.5 kg mass. Even so, the weight of the ball is more similar 55 N. That's more than than the buoyant strength of the h2o it displaced, and then information technology sinks.
A embankment ball may have the same book as a bowling ball, but it has a much smaller mass. When y'all a beach ball in a tub of water, information technology displaces the mass of water equal to its ain mass—nearly 0.01 kg. If you were to try to button the beach ball down and displace more water, the water would push back with a force greater than the weight of the beach brawl. The button of the h2o keeps the embankment ball adrift.
Buoyancy is the upward strength we need from the water to stay afloat. Buoyant forces are why we feel and then much lighter when nosotros are in a swimming pool. Our bodies are mostly water, and so our density is fairly close to that of h2o. Considering of this, an average person needs only a piffling bit extra buoyancy to float. A life jacket provides this extra elevator.
Changing Density
You can alter the density of a substance by heating information technology, cooling it, or past adding something to it. If an object sinks in water, it's because the object has a college density than the h2o. At that place are two possible ways to make that object float, yet:
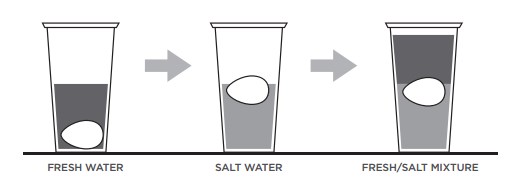
- Increase the density of the water and so that the water becomes denser than the object. For instance, an egg will usually sink in a glass of h2o, because it is denser than water. Adding table salt to the water increases the density of the water, allowing the egg to bladder. This experiment likewise works with people, but yous need a lot of salt (try the ocean, or even better, the Dead Sea !)
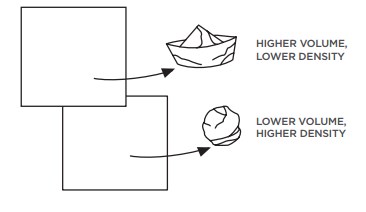
- Increase the volume of the object so that the object becomes less dense than the water. A slap-up instance of this is ice floating in water. Ice is formed by freezing water. When information technology freezes, it increases in book equally the h2o molecules move further autonomously to accommodate the lattice structure of ice. Considering the ice is now less dumbo than h2o, it floats. This phenomenon besides explains why ships bladder even though they are made of steel. A transport is built in such a style that it encloses large amounts of open up space. The ship even so displaces its weight in water, only because of the manner the ship is synthetic, information technology takes up more infinite than the volume of the water it displaces, so it floats.
Vocabulary
Archimedes: Greek mathematician, physicist, engineer, inventor and astronomer (c. 287 BC–c. 212 BC).
Archimedes' principle: Whatever object wholly or partially immersed in a fluid is buoyed by a force equal to the weight of the fluid that is displaced by the object. In other words, the buoyancy is equal to the weight of the displaced fluid.
buoyancy: The up force that a fluid exerts on an object less dense than itself; the ability to float.
density: How closely packed together the molecules of an object or substance are.
displace: To push out of the way. For case, when an object goes into water, it displaces the water.
immiscible: Unable to be mixed together, like oil and h2o.
ironwood: The proper noun for a large number of woods that accept a reputation for hardness and high density.
mass: The corporeality of affair in a given space.
matter: The substance that makes up all physical things.
pumice stone: Lava froth known for its small mass and low density, despite looking like a rock.
weight: A measure out of the forcefulness of gravity on an object.
volume: The amount of space a substance or object takes up.
Other Resources
BrainPOP | Science | Thing & Chemistry | Measuring Matter
EDinformatics | Mass, Volume, Density
WatchKnowLearn.org | Buoyancy and Density
ProTeacher Collection | Density
Source: https://www.scienceworld.ca/resource/floaters-and-sinkers/
0 Response to "Can a Higher Density Material Float on a Lower Density Material"
Postar um comentário